Description
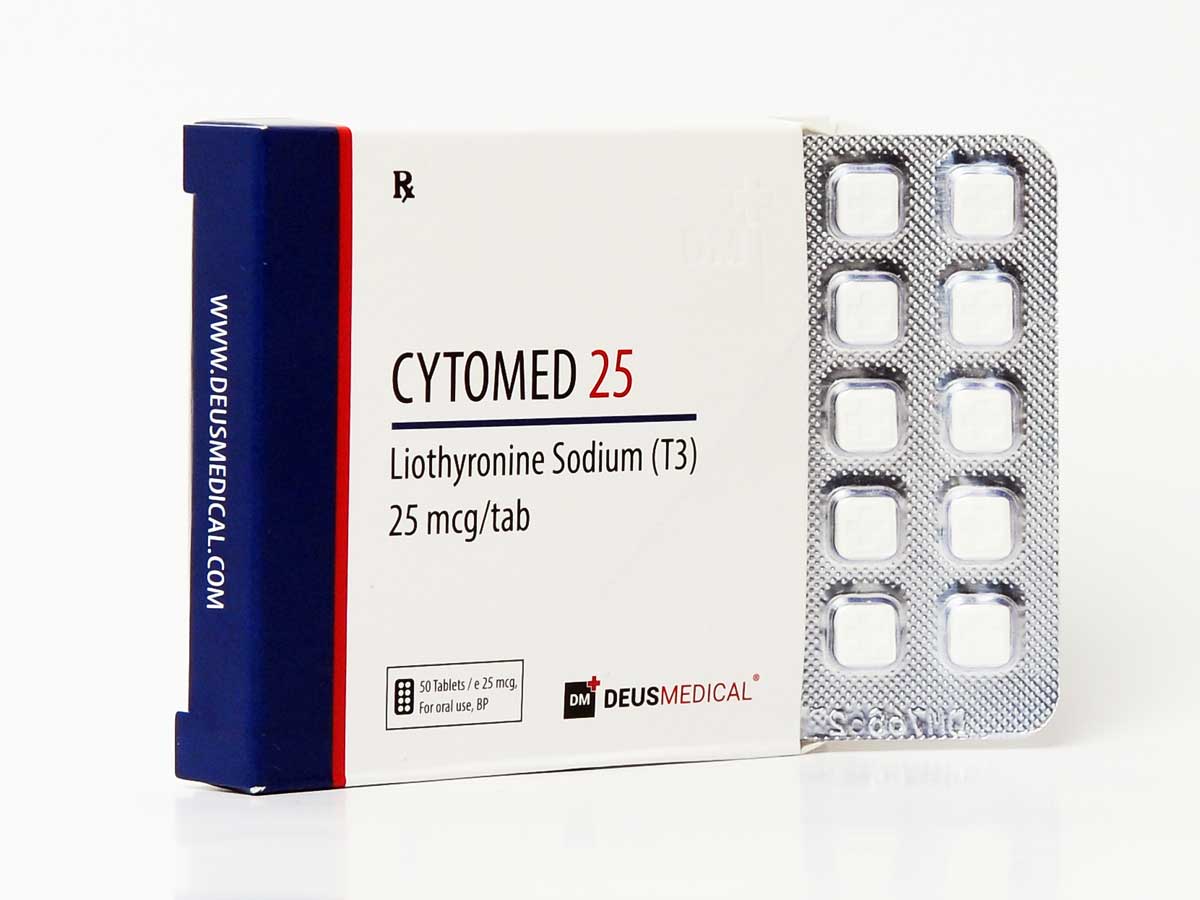
CYTOMED 25 DEUS MEDICAL
Product Description
CYTOMED 25 by Deus Medical is a high-quality synthetic thyroid hormone, specifically designed to aid in fat loss and enhance metabolism. It contains the active ingredient Liothyronine Sodium, which is a synthetic form of the thyroid hormone triiodothyronine (T3).
Specific Details
- Active Ingredient: Liothyronine Sodium
- Brand: Deus Medical
- Strength: 25mcg per tablet
- Pack Size: 100 tablets
- Form: Oral tablets
Features and Benefits
- Promotes fat loss by increasing metabolic rate
- Enhances energy levels and stamina
- Improves protein synthesis and utilization
- Boosts overall metabolism
- Helps in maintaining lean muscle mass during cutting phases
Possible Side Effects
While CYTOMED 25 is generally well-tolerated, some individuals may experience side effects such as:
- Increased heart rate
- Excessive sweating
- Insomnia
- Tremors
- Anxiety
Usage and Dosage
For beginners, it is recommended to start with a low dosage of 25mcg per day and gradually increase it by 25mcg every 3-4 days until reaching the desired dosage. Experienced athletes can start with a higher dosage of 50mcg per day.
Benefits for the Buyer
- High-quality product from Deus Medical
- Effective fat loss and metabolism enhancement
- Improved energy levels and stamina
- Preserves lean muscle mass during cutting phases
- Convenient and discreet online purchase
Why Buy from Anabolicsteroidsshop.com in England
- Wide range of genuine and high-quality products
- Secure and discreet packaging
- Fast and reliable delivery
- Competitive prices
- Excellent customer service


Reviews
There are no reviews yet.